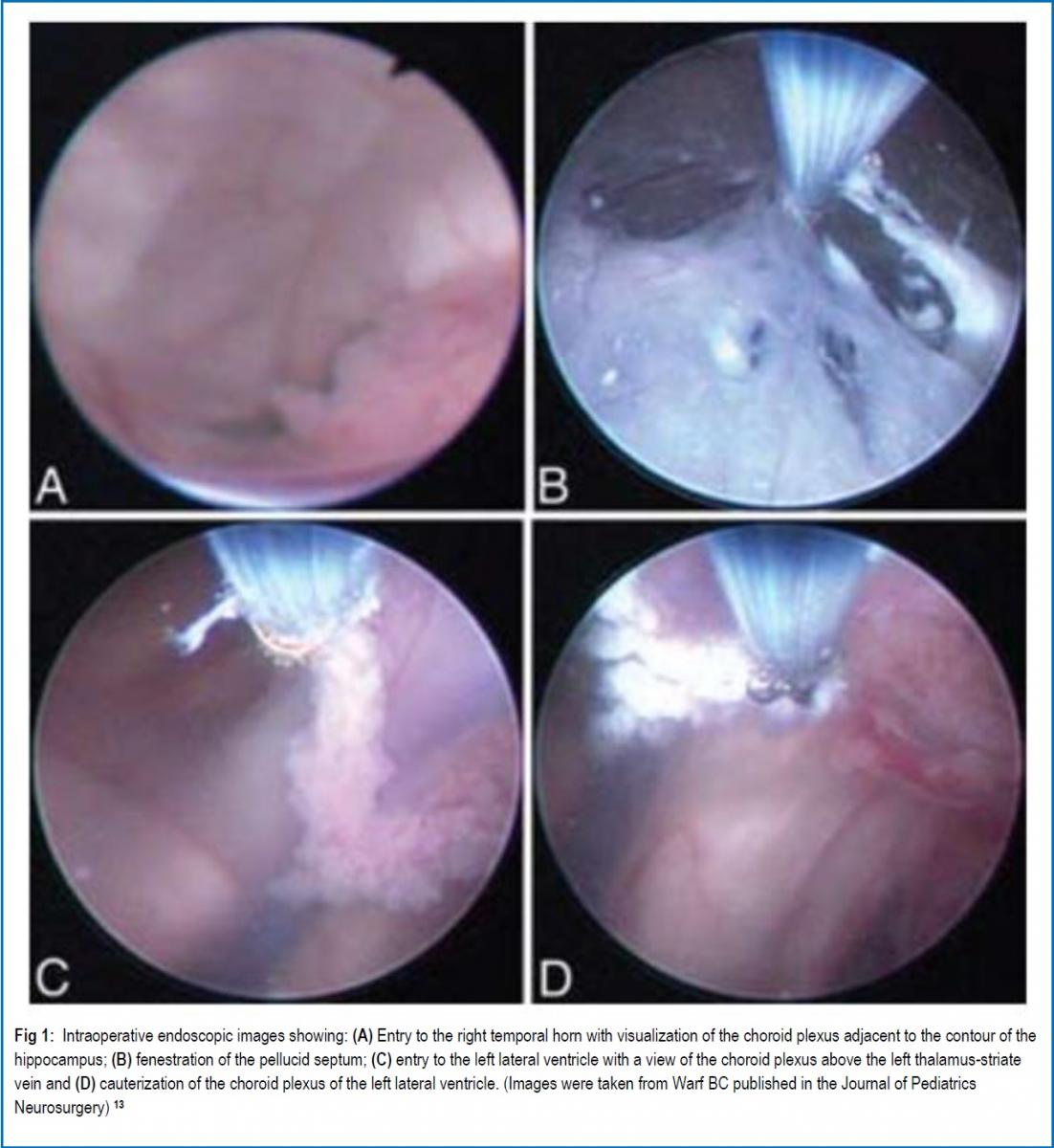
INTRODUCTION
Choroid plexus coagulation (CPC) is a procedure that arises from the historical attempt to treat hydrocephalus, 1-7 however, it is replaced by bypass therapies as the standard of treatment due to better results.8 Complications of CSF shunts It can be serious, so any method of reducing dependence on these can be beneficial for patients, especially in social and health contexts with difficult access to care and a health system with significant financial limitations. Up to 14% failure of CSF shunts is reported per month and 20 to 50% in the first year in the general population with hydrocephalus.9
METHODS
PubMed was searched for the terms (choroid plexus coagulation) OR (choroid plexus cauterization). Those studies published since 1990 were included so that they were relevant to current clinical practice. All studies evaluating the efficacy of CPC as an isolated procedure for treating hydrocephalus were included.
A detailed review of the references of the selected papers provided additional studies. Historical reviews about the procedure and exclusive review articles were excluded.
All patients in whom CPC was associated with another concomitant procedure, or whose aim was not to evaluate it as a therapy for hydrocephalus, were excluded from the results. Results corresponding to the same patient in different studies were excluded from the statistical analysis.
A total of 329 results were found, of which 12 were included in the data processing of the results of the review.
The chi2 test was used to determine the association between variables and the outcome in a population with an assumed normal distribution and Fisher's exact test when associating a population in which normality of the sample distribution could not be assumed.
RESULTS
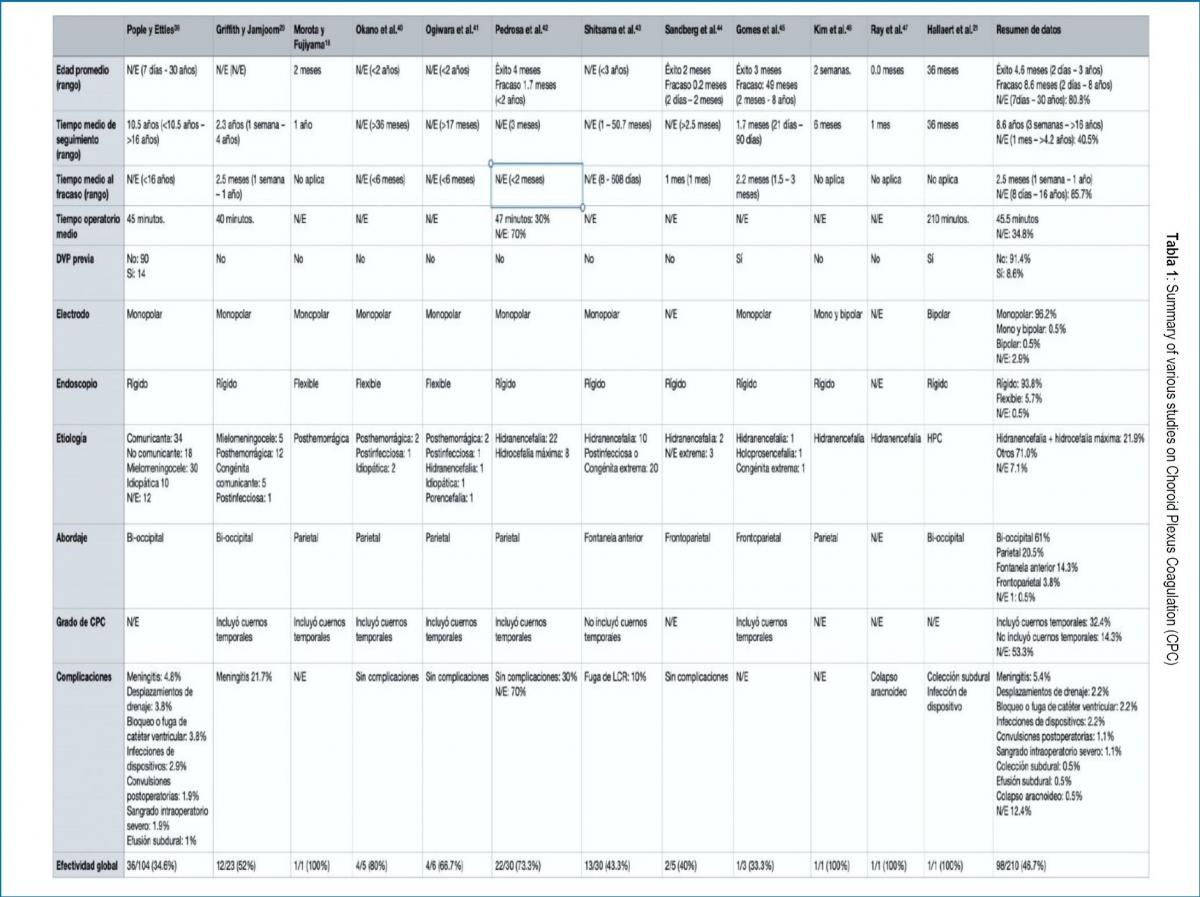
The total N evaluated in this review was 210 patients, with an overall success of CPC as a treatment for hydrocephalus of 46.7%. It was defined as the success of the procedure that the patients did not need another intervention to control hydrocephalus at the end of the follow-up. The data from the primary studies are summarized in Table 1.
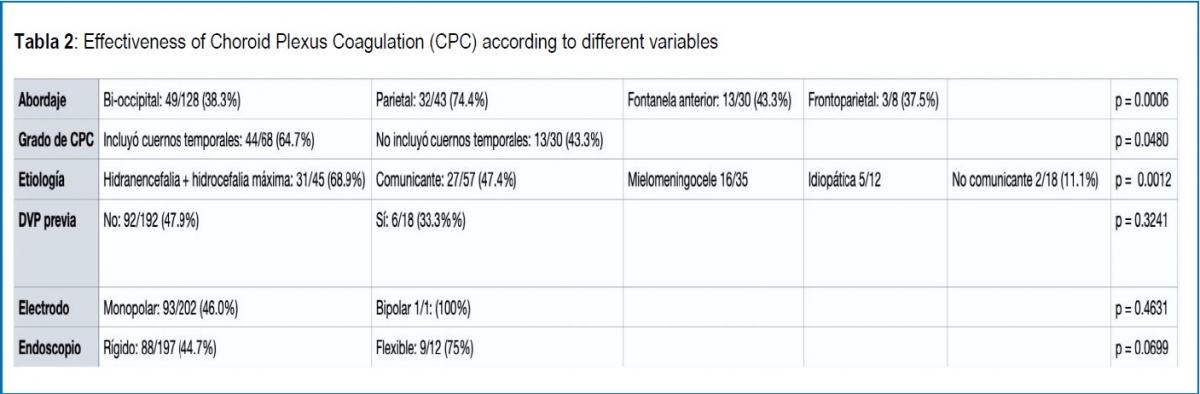
The studies do not provide sufficient information on sex, head circumference (CC), rate of CC progression, or age at the time of surgery to determine an association with the effectiveness of the treatment. Table 2 shows the effectiveness according to available variables.
The etiology, approach, and degree of CPC were associated with the effectiveness of the procedure. The greatest effectiveness was reported in patients with hydranencephaly or maximum hydrocephalus (almost hydranencephaly, little cortical remnant) (68.9%, p = 0.001276), when a parietal approach was used (74.4%, p = 0.000604), and when the plexus was included of the temporal horns (64.7%, p = 0.048068). In extreme hydrocephalus (very thin cerebral cortex present, any etiology) the effectiveness was 37.5%. Ogiwara et al.41 reported hydranencephaly as an etiology in 1 patient but did not specify whether the procedure was successful or not, so it was not included in the effectiveness analysis when comparing etiology.
On the other hand, the type of electrode (p = 0.4631), the type of endoscope (p = 0.0699), and the presence of a previous ventriculoperitoneal shunt (VPS) (p = 0.3241) were not significantly associated with the effectiveness of CPC.
DISCUSSION
Hydrocephalus is a pathology with a reported mortality of up to 80% without treatment,49 therefore, in medical practice, the implementation of effective therapies is essential. CPC reappears as an alternative treatment for hydrocephalus due to the inherent risks of a ventricul0peritoneal shunt (VPS).9 The main use of the CPC at present is together with the ETV to increase the effectiveness of the latter.10-17 However, the CPC also has a role as an isolated procedure.
The mechanism by which hydrocephalus develops is not fully elucidated, but the most widely accepted is that it would be generated by an imbalance between CSF production and absorption.23 The traditional model of CSF net flow is based on CSF production in the choroid plexus, its subsequent distribution through the subarachnoid space, and finally its absorption in the arachnoid villi.
The presence of the choroid plexus has been reported as necessary for the production of ventriculomegaly.24-26 Other studies have shown that in a communicating hydrocephalus model the ventricles fail to expand in the absence of normal pulsatility of the choroid plexus.27 Furthermore, the role of increased secretion of CSF by the choroid plexus in the development of hydrocephalus is reported.28 Hypotheses suggest a decreased absorption of CSF in children, in part due to immature arachnoid villi more sensitive to changes in intracranial pressure (ICP), with a large decrease in resorption compared to slight increases in ICP.29-32 The latter, associated with a skull with greater compliance, may mask the severity of the condition.33 These studies represent, in part, the theoretical basis for CPC as a treatment for hydrocephalus.
In response to this model, a theory arises that tries to explain scenarios that the classical model cannot fully respond to, such as hydrocephalus without intracranial hypertension (ICP), arrested hydrocephalus, hydrocephalus without a transependymal pressure gradient, and CSF circuit obstruction without hydrocephalus. The new hydrodynamic model proposes that the interstitial fluid (IF) and the CSF volume (water) constitute a functional unit and are regulated by changes in the hydrostatic and osmotic pressures of microvessels throughout the entire central nervous system (CNS). The continuous turnover of the IF – CSF (water) volume would be created by the filtration of water through the walls of the arterial capillaries at high hydrostatic pressure with plasma retention of osmolytes (reflection coefficient of the main electrolytes Na + and Cl- is 0.98) and the reabsorption of water from the interstitium into the venous capillaries and postcapillary venules by the resulting osmotic back pressure. The changes in CSF volume would depend on physiological and pathophysiological processes that cause differences in osmolarity between the CNS compartments.34
This theory is supported by the development of cerebral edema under conditions of decreased blood osmolarity in relation to the brain parenchyma and the CSF.35, 36 Conversely, hyperosmolar solutions are used in clinical practice to lower intracranial pressure through the osmotic movement of water from the brain tissue. 34 Water accumulation in brain tissue has also been observed due to increased post-trauma osmolarity and in ischemia.37, 38 According to this theory, hydrocephalus would be a pathological state rather than pathology in itself and its origin would not lie mainly in an overproduction of CSF in the choroid plexuses, an alteration in its circulation, or an absorption insufficient at the level of the arachnoid villi, but rather the result of various pathophysiological processes that affect They would regulate the extracellular volume as in other parts of the body.34The CPC would be intended to help the hydrodynamic balance of the CSF, but it is difficult to know if the CPC has a long-term effect in reducing the production of CSF and thus helps the dynamic balance of the CSF, or during the time that CPC works, allow the body to increase the CSF reabsorption.18, 23.
Studies in the population with hydrocephalus show variable success rates according to the characteristics of the patients (33.3% - 73.3%) with an overall effectiveness rate of 46.7%.
All the studies included in the analysis were retrospective studies, most of them also with small population sizes. Selection bias is high; few studies have a patient selection system to use this procedure. Furthermore, significant losses to follow-up are reported without assigning them as failures. To minimize confounding bias, the statistical significance of different factors was calculated, so that no greater weight was assigned to a specific one or to the association with another.
Pople and Ettles 39 describe in their series that in those patients with rapidly progressive hydrocephalus (tense fontanelle) CPC was less effective (11% vs 46%, p = 0.03), but this represents an important measurement bias because it represents the evolution of a parameter by means of a subjective clinical estimation at a given time. Shitsama et al.43 describe the same effect (p = 0.045), but how this conclusion was reached is not mentioned. The positive results found in this group of patients could in part be due to milder hydrocephalus, with even a possible spontaneous arrest of disease progression independent of the procedure. However, it is not possible to draw a conclusion without knowing the progression of a certain parameter over time until the time of surgery and establish cut-off points to separate the "slowly" from the "rapidly progressive" one. In Figure 2, an original algorithm is proposed to decide to perform a CPC, based on the variables studied in this review.
In a population with communicating hydrocephalus, success rates are higher than in non-communicating hydrocephalus (47.4% vs 11.1%) and like myelomeningocele (45.7%). This may have to do with a pathophysiological explanation since in non-communicating hydrocephalus the main problem would be determined by an obstruction at the level of the subarachnoid space and/or in the ventricular system, so plexus ablation would not have a major role in your resolution. It is for this reason that CPC would not be indicated in obstructive hydrocephalus.
The etiology was significantly associated with the effectiveness of the procedure, the latter being the highest in patients with hydranencephaly or maximum hydrocephalus (68.9%, p = 0.001276). It is important not to confuse hydranencephaly or maximum hydrocephalus with extreme hydrocephalus, in the latter group the procedure was successful only in 37.5% of the cases. Hydranencephaly is a condition with high mortality, mostly in the first two years of life, in which the main cause of death is pulmonary complications and infections. 50 An absent cortex or a remnant of it means that in these patients a CSF shunt has a greater risk of complications, mainly CSF leak, shunt dysfunction, and skin damage due to thinning of the “Scalp”.19, 20 For this reason, CPC emerges as a management option for this complex type of patients with the aim of reducing complications associated with interventions for the treatment of hydrocephalus, reducing the number of interventions and improving the quality of life of patients.
Even though multiple studies have established that most of the CSF would be produced in the ependyma, 51-53 in these patients, in particular, the lack of brain parenchyma as a CSF producer and the greater ease of performing the procedure due to anatomical distortion, mainly absence or thinning of the septum pellucid for the coagulation of the contralateral plexus, could be the cause of the favorable results. The only prospective study 41 that compared CPC with VPS for the treatment of hydranencephaly showed that there is no significant difference between their success rates. In addition, it showed that the cost associated with VPS is more than double that of a CPC, due to the supplies used, the interventions, and the total number of extra bed days used. In contexts of limited resources, the latter can be decisive when choosing a procedure.
CPC occurs in the treatment of choroid plexus hyperplasia (CPH), a rare pediatric disorder that would always present with increased CSF production.21 This increased CSF production would determine a higher risk of complication of a shunt, a review study 21 showed that 16 of 17 patients with CPH developed ascites associated with VPS. In most cases, the final procedure is a plexectomy, but this is a procedure with a high risk of bleeding,22 so CPC appears like a safer option. The evidence is scant, and they are only case reports. The diagnosis is suspected with a bilaterally enlarged choroid plexus on preoperative MRI and is confirmed by histology with normal plexus, without mitotic figures or elevated Ki67. In all cases, CPC is presented as a secondary procedure for the treatment of CPH when VPS has failed. In the only patient in whom CPC was evaluated as a definitive procedure to treat hydrocephalus secondary to HPC, the procedure was successful.21 In the other studies found, no attempt was made to leave the patients free of a shunt, therefore, we cannot draw conclusions regarding the effectiveness of CPC as management of hydrocephalus in this group and were also not included in the results of this review. In these last-mentioned studies, it was found that in 3 patients 54-56 CSF production decreased and in 1 case 57 it remained the same. Considering the quality of the available evidence and the low probability of a prospective study due to the epidemiology of this pathology, CPC may be useful as a definitive treatment for this disorder, and with more security, it may also be useful in reducing the risk of development of ascites when associated with VPS, as well as avoiding the risks associated with a plexectomy.
The degree of CPC is associated with the effectiveness of the procedure, with a higher success rate in those that included coagulation of the choroid plexus of the temporal horns (64.7 vs 43.3%, p = 0.048068). This is probably explained by greater control of CSF production in the choroid plexuses; therefore, extensive cauterization of the plexuses is recommended, including both plexuses corresponding to the temporal horns.
The use of a flexible endoscope reported a higher success rate than the rigid endoscope (75% vs 44.7%, p = 0.0699). This difference close to statistical significance can be explained by the small sample size in the flexible endoscope group (5.7% of the total sample). The use of a flexible endoscope would be associated with success because it would technically facilitate the coagulation of a greater proportion of the plexus in relation to a rigid endoscope.58,59.
The type of approach was also significantly associated with the effectiveness of the procedure (p = 0.000604). Maximum effectiveness was achieved through a parietal approach (74.4%), while the other approaches had similar success rates (37.5 - 43.3%). One of the advantages of the parietal approach is that it does not need more than one entry point, unlike the bi-occipital approach in which 2 burr holes are required, which in certain cases it is even mentioned that they were placed with the help of Neuronavigation, a resource not always available.
Like what happened with the almost exclusive use of a rigid endoscope, the monopolar electrode was the choice in 96.2% of the sample. Thus, the type of electrode was not significantly associated with the effectiveness of the procedure (p = 0.4631). In the only patient who exclusively used a bipolar electrode, the procedure was successful. With the present results, an assessment of the role of the bipolar electrode in CPC cannot be made, but it is not ruled out that it may be used in centers with experience in its use. In other cases, it is prudent to proceed with a monopolar electrode due to the bulk of published evidence with its use.
The presence of a previous VPS was not significantly associated with a lower CPC effectiveness (p = 0.3241). This possibly due to processes in which the overproduction of CSF by the plexus prevailed and that the derivation was not able to balance.
CONCLUSION
Isolated CPC is a viable treatment option for hydranencephaly, choroid plexus hyperplasia, and to a lesser extent for communicating hydrocephalus with non-tense fontanelle. Factors associated with its success are a greater degree of coagulation of the choroid plexus, a parietal approach, and probably the use of a flexible endoscope.
Finally, more prospective evidence is still needed that directly compares CPC with bypass therapies and evaluates long-term functional and neurocognitive outcomes when possible, as well as patient and family satisfaction and quality of life. The latter is of special importance in patients who have a reserved survival prognosis such as hydranencephaly. All this, with the objective to offer the most information to the family when making the decision about a procedure to be carried out.
REFERENCES
- Dandy WE. Extirpation of the choroid plexus of the lateral ventricles in communicating hydrocephalus. Ann Surg 1918;68: 569-579.
- Dandy WE. The operative treatment of communicating hydrocephalus. Ann Surg 1938;108: 194-202.
- L'Espinasse VL: in Davis (ed): Neurological Surgery. Philadelphia, Lea & Febiger, 1943, ed 2, p 442.
- Scarff JE. Evaluation of treatment of hydrocephalus. Results of third ventriculostomy and endoscopic cauterization of choroid plexuses compared with mechanical shunts. Arch Neurol 1966; 14:382-391.
- Putnam TJ. Treatment of hydrocephalus by endoscopic coagulation of choroid plexuses: description of a new instrument. N Engl J Med 1934; 210:1373– 1376.
- Davidoff LM. Hydrocephalus and hydrocephalus with meningocele: Their treatment by choroid plexectomy. Surg Clin North Am 1948; 28:416-431.
- Sachs E. Hydrocephalus: An analysis of 98 cases. J Mt Sinai Hosp 1942; 9:767-791
- Milojević AJ, Radojčić BS, Meljnikov IĐ. Hydrocephalus – History of surgical treatment over the centuries. Sanamed 2012; 7(2): 119–125.
- Wu Y, Green NL, Wrensch MR, et al. Ventriculoperitoneal shunt complications in California: 1990 to 2000. Neurosurgery. 2007; 61(3):557-563.
- Warf BC. Comparison of endoscopic third ventriculostomy alone and combined with choroid plexus cauterization in infants younger than 1 year of age: a prospective study in 550 African children. J Neurosurg 2005; 103: 475-481.
- Warf B, Ondoma S, Kulkarni A, et al. Neurocognitive outcome, and ventricular volume in children with myelomeningocele treated for hydrocephalus in Uganda. J Neurosurg Pediatr 2009;4:564-570.
- Warf BC. Congenital idiopathic hydrocephalus of infancy: the results of treatment by endoscopic third ventriculostomy with or without choroid plexus cauterization and suggestions for how it works. Childs Nerv Syst 2013; 29:935-940.
- Warf BC, Campbell JW. Combined endoscopic third ventriculostomy and choroid plexus cauterization as primary treatment of hydrocephalus for infants with myelomeningocele: long-term results of a prospective intent-to-treat study in 115 East African infants. J Neurosurg Pediatr 2008;2:310-316.
- Warf BC, Campbell JW, Riddle E. Initial experience with combined endoscopic third ventriculostomy and choroid plexus cauterization for post-hemorrhagic hydrocephalus of prematurity: the importance of prepontine cistern status and the predictive value of FIESTA MRI imaging. Childs Nerv Syst. 2011;27(7):1063‐1071.
- Warf BC, Dewan M, Mugamba J. Management of Dandy-Walker complex-associated infant hydrocephalus by combined endoscopic third ventriculostomy and choroid plexus cauterization. J Neurosurg Pediatr 2011;8:377-383.
- Warf BC, Stagno V, Mugamba J. Encephalocele in Uganda: ethnic distinctions in lesion location, endoscopic management of hydrocephalus, and survival in 110 consecutive children. J Neurosurg Pediatr 2011; 7: 88-93.
- Warf BC, Tracy S, Mugamba J. Long-term outcome for endoscopic third ventriculostomy alone or in combination with choroid plexus cauterization for congenital aqueductal stenosis in African infants. J Neurosurg Pediatr 2012; 10: 108-111.
- Morota N, Fujiyama Y. Endoscopic coagulation of choroid plexus as a treatment for hydrocephalus: indication and surgical technique. Childs Nerv Syst 2004; 20:816–820.
- Wellons JC 3rd, Tubbs RS, Leveque JC, et al. Choroid plexectomy reduces neurosurgical intervention in patients with hydranencephaly. Pediatr Neurosurg. 2002; 36(3):148‐152.
- Griffith HB, Jamjoom AB. The treatment of childhood hydrocephalus by choroid plexus coagulation and artificial cerebrospinal fluid perfusion. Br J Neurosurg. 1990;4(2):95-100.
- Hallaert GG, Vanhauwaert DJ, Logghe K, et al. Endoscopic coagulation of choroid plexus hyperplasia. J Neurosurg Pediatr. 2012;9(2):169‐177.
- Piastra M, Di Rocco C, Tempera A, et al.Massive blood transfusion in choroid plexus tumor surgery: 10-years’ experience. J Clin Anesth 2007;19:192–197.
- Orešković D, Radoš M, Klarica M: Role of choroid plexus in cerebrospinal fluid hydrodynamics. Neuroscience 2017; 354:69– 87.
- Bering EA Jr. Circulation of the cerebrospinal fluid. Demonstration of the choroid plexuses as the generator of the force for the flow of fluid and ventricular enlargement. J Neurosurg 1962; 19:405–413.
- Egnor M, Zheng L, Rosiello A, et al. A model of pulsations in communicating hydrocephalus. Pediatr Neurosurg 2002; 36:281–303.
- Wilson CB, Bertan V. Interruption of the anterior choroidal artery in experimental hydrocephalus. Arch Neurol 1967; 17:614– 619.
- Di Rocco C, Pettorossi VE, Caldarelli M, et al. Communicating hydrocephalus induced by mechanically increased amplitude of the intraventricular cerebrospinal fluid pressure: experimental studies. Exp Neurol 1978; 59:40–52.
- Karimy JK, Duran D, Hu JK, et al. Cerebrospinal fluid hypersecretion in pediatric hydrocephalus. Neurosurg Focus. 2016;41(5): E10.
- Grunert P, Charalampaki P, Hopf N, Filippi R. The role of the third ventriculostomy in the management of obstructive hydrocephalus. Minim Invasive Neurosurg 2003; 46:16–21.
- Javadpour M, Mallucci C. The role of neuroendoscopy in the management of tectal gliomas. Childs Nerv Syst. 2004;20(11-12):852‐857.
- Teo C, Jones R. Management of hydrocephalus by endoscopic third ventriculostomy in patients with myelomeningocele. Pediatr Neurosurg. 1996;25(2):57‐63.
- Oi S, Di Rocco C. Proposal of “evolution theory in cerebrospinal fluid dynamics” and minor pathway hydrocephalus in developing immature brain. Childs Nerv Syst 2006; 22:662–669.
- Zandian A, Haffner M, Johnson J, et al. Endoscopic third ventriculostomy with/without choroid plexus cauterization for hydrocephalus due to hemorrhage, infection, Dandy-Walker malformation, and neural tube defect: a meta-analysis. Childs Nerv Syst. 2014;30(4):571‐578.
- Orešković D, Klarica M. Development of hydrocephalus and classical hypothesis of cerebrospinal fluid hydrodynamics: facts and illusions. Prog Neurobiol. 2011;94(3):238‐258.
- Go KG. The normal and pathological physiology of brain water. Adv. Tech. Stand. Neurosurg. 1997; 23:47–142.
- Verbalis JG. Brain volume regulation in response to changes in osmolality. Neuroscience. 2010;168(4):862-870.
- Hossmann KA. The pathophysiology of ischemic brain swelling. 1985, In: Inaba, Y., Klatzo, I., Spatz, M. (Eds.), Brain Edema. Springer-Verlag, Berlin, Heildeberg, New York, Tokyo, pp. 365–384.
- Kawamata T, Mori T, Sato S, Katayama Y. Tissue hyperosmolality and brain edema in cerebral contusion. Neurosurg Focus. 2007;22(5): E5.
- Pople IK, Ettles D. The role of endoscopic choroid plexus coagulation in the management of hydrocephalus. Neurosurgery 1995;36(4):698‐702.
- Okano A, Ogiwara H. Long-term follow-up for patients with infantile hydrocephalus treated by choroid plexus coagulation. J Neurosurg Pediatr 2018; 22(6):638–645.
- Ogiwara H, Uematsu K, Morota N. Obliteration of the choroid plexus after endoscopic coagulation. J Neurosurg Pediatr. 2014;14(3):230‐233.
- Pedrosa HA, Lemos SP, Vieira C, et al. Choroid plexus cauterization on the treatment of hydranencephaly and maximal hydrocephalus. Childs Nerv Syst. 2017;33(9):1509‐1516.
- Shitsama S, Wittayanakorn N, Okechi H, Albright L. Choroid plexus coagulation in infants with extreme hydrocephalus or hydranencephaly. J Neurosurg Pediatr 14:55–57, 2014.
- Sandberg DI, Chamiraju P, Zoeller G, et al. Endoscopic choroid plexus coagulation in infants with hydranencephaly or hydrocephalus with a minimal cortical mantle. Pediatr Neurosurg 2012;48(1):6‐12.
- Gomes FL, Loduca RD, Homa MN, Collange NZ. Endoscopic coagulation of choroid plexus in three children with severely advanced forms of hydrocephalus. J Neurol Surg A Cent Eur Neurosurg 2015; 76 (1): 25‐29.
- Kim SY, Cho JH, Kim KH. Endoscopic Coagulation of Choroid Plexus in Hydranencephaly. J Korean Neurosurg Soc 2014;55(6):375-378.
- Ray C, Mobley J, Thompson M, Nagy L. Hydranencephaly: Considering Prolonged Survival and Treatment by Endoscopic Choroid Plexus Coagulation. Turk Neurosurg. 2015;25(5):788‐792.
- Malheiros JA, Trivelato FP, Oliveira MM, et al. Endoscopic choroid plexus cauterization versus ventriculoperitoneal shunt for hydranencephaly and near hydranencephaly: a prospective study. Neurosurgery 2010; 66:459–464; discussion 464.
- Laurence KM, Coates S. The natural history of hydrocephalus. Detailed analysis of 182 unoperated cases. Arch Dis Child. 1962;37(194):345‐362.
- Merker B. Life expectancy in hydranencephaly. Clin Neurol Neurosurg 2008;110(3):213–214.
- Milhorat TH, Hammock MK, Chien T, Davis DA. A normal rate of cerebrospinal fluid formation five years after bilateral choroid plexectomy. Case report. J Neurosurg. 1976;44(6):735-739.
- Pollay M, Curl F. Secretion of cerebrospinal fluid by the ventricular ependyma of the rabbit. Am J Physiol. 1967;213(4):1031-1038.
- Sato O, Bering EA. Extra-ventricular formation of cerebrospinal fluid. No To Shinkei. 1967;19(9): 883-885.
- Tamburrini G, Caldarelli M, Di Rocco F, et al: The role of endoscopic choroid plexus coagulation in the surgical management of bilateral choroid plexuses hyperplasia. Childs Nerv Syst 2006; 22:605–608.
- Philips MF, Shanno G, Duhaime AC. Treatment of villous hypertrophy of the choroid plexus by endoscopic contact coagulation. Pediatr Neurosurg 1998; 28:252–256.
- Bucholz RD, Pittman T. Endoscopic coagulation of the choroid plexus using the Nd: YAG laser: initial experience and proposal for management. Neurosurgery 1991; 28:421–427.
- Kasper J, Krause M, Siekmeyer M, Gräfe D, Meixensberger J, Wilhelmy F. Choroid plexus coagulation in trisomy 9 mosaic-related hydrocephalus-a case report [published online ahead of print, 2020 May 8]. Childs Nerv Syst. 2020;10.1007/s00381-020-04643-1.
__________________________________________
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Authors Contributions
Conception and design: Smoquina, Zulueta. Drafting the article: Smoquina. Critically revising the article: Smoquina. Reviewed submitted version of manuscript: Smoquina, Zulueta. Approved the final version of the manuscript on behalf of all authors: Smoquina.
Correspondence
Stefano Smoquina. Neurosurgery Fellow. University of Valparaíso, Chile. E-mail: stefano.smoquina@gmail.com